About
About ENDOMETRIN
Prescribe ENDOMETRIN for your patients to support embryo implantation and early pregnancy by supplementation of corpus luteal function as part of an assisted reproductive technology (ART) treatment program for infertile women.1

In a US IVF trial with more than 1,200 patients, ENDOMETRIN was proven safe and effective.2
Study Design
ENDOMETRIN has been a part of IVF protocols since 2007.1
Support
Administration
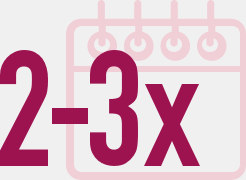
Adaptable dosing
Each ENDOMETRIN insert supplies 100 mg of progesterone and comes with its own single-use disposable applicator for sterile insertion into the vagina, 2 or 3 times a day, for up to 10 weeks, as specified.1

How to Use ENDOMETRIN
For full step-by-step instructions on how to use ENDOMETRIN, download the how-to guide for your patients.1

Savings
ENDOMETRIN savings
While the ENDOMETRIN Patient Savings Program is no longer available as of 5/31/23, Ferring is committed to supporting access to treatments. Please visit Patient Resources - Ferring Pharmaceuticals (ferringfertility.com) or speak with your healthcare provider to learn about other options that may be available.
Access
Formulary Lookup Tool
ENDOMETRIN® (progesterone) Vaginal Insert
Indication
ENDOMETRIN® (progesterone) Vaginal Insert is indicated to support embryo implantation and early pregnancy by supplementation of corpus luteal function as part of an Assisted Reproductive Technology (ART) treatment program for infertile women.
Important Safety Information
- ENDOMETRIN should not be used in individuals with any of the following conditions: previous allergic reactions to progesterone or any of the ingredients of ENDOMETRIN, undiagnosed vaginal bleeding, known missed abortion or ectopic pregnancy, liver disease, known or suspected breast cancer, active arterial or venous thromboembolism or severe thrombophlebitis, or a history of these events.
- The physician should be alert to earliest signs of myocardial infarction, cerebrovascular disorders, arterial or venous thromboembolism (venous thromboembolism or pulmonary embolism), thrombophlebitis, or retinal thrombosis. ENDOMETRIN should be discontinued if any of these are suspected.
- Patients with a history of depression need to be closely observed. Consider discontinuation if symptoms worsen.
- ENDOMETRIN should not be recommended for use with other vaginal products (such as antifungal products) as this may alter progesterone release and absorption from the vaginal insert.
- The most common adverse reactions reported (greater than 5%) were post-oocyte retrieval pain, abdominal pain, nausea, and ovarian hyperstimulation syndrome.
You are encouraged to report negative side effects of prescription drugs to FDA. Visit www.FDA.gov/medwatch, or call 1.800.FDA.1088.
Please see full Prescribing Information.
ENDOMETRIN® (progesterone) Vaginal Insert
Indication
ENDOMETRIN® (progesterone) Vaginal Insert is indicated to support embryo implantation and early pregnancy by supplementation of corpus luteal function as part of an Assisted Reproductive Technology (ART) treatment program for infertile women.
Important Safety Information
- ENDOMETRIN should not be used in individuals with any of the following conditions: previous allergic reactions to progesterone or any of the ingredients of ENDOMETRIN, undiagnosed vaginal bleeding, known missed abortion or ectopic pregnancy, liver disease, known or suspected breast cancer, active arterial or venous thromboembolism or severe thrombophlebitis, or a history of these events.